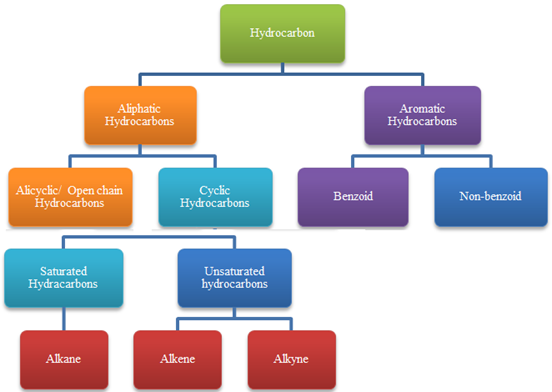
Hydrocarbons
- Compounds of carbon and hydrogen.
- Classification of Hydrocarbons:
Alkane
- Open chain saturated hydrocarbon with general formula (CnH2n+2).
- All the C atoms are single bonded i.e. sp3 hybridised.
Conformations of Alkane
- Conformations are the different arrangement of atoms that can be converted into one another by rotation about single bonds.
- Eclipsed Conformation: H atoms on two adjacent carbon atoms are closest to each other i.e. dehydral angle is 0.

- Staggered Conformation: H atoms on two adjacent carbon atoms are farthest to each other i.e. dehydral angle is 60.


Preparation of Alkanes:
- Reduction of Alkyl Halides:
RX + Zn: + H+ → RH + Zn2+ + X-
4RX + LiAlH4 → 4RH + LiX + AlX3 (X≠ F)
RX + (n - C4H9)3 SnH → R-H + (n - C4H9)3 SnX
- Grignard Reagent:
?

- Hydrogenation of Alkenes:

- Wurtz Reaction:
2RX + 2Na → R-R + 2NaX
2Na + 2CH3CH2CH2Cl → CH3CH2CH2CH2-CH2CH3 + 2NaCl
- Corey House Reaction:
- Decarboxylation of a mixture of the sodium salt of a carboxylic acid:
RCOONa +NaOH(CaO) → RH + Na2CO3
- Kolbe's electrolytic method:
2 RCOOK + 2H2O → R-R + 2CO2 + H2+ 2KOH
Chemical Properties of Alkane
- Direct Halogenation
RH + X2→ RX + HX
Order of Reactivity of X2: F2 > Cl2 > Br2; I2 does not react
?a. Initiation Step
Cl-Cl  2Cl.
2Cl.
 2Cl.
2Cl.
b. Propagation Step
H3C-H +Cl. → H3C. + H-Cl
H3C. + Cl-Cl → H3C-Cl +Cl.
c. Termination Step
Cl. + Cl. →Cl-Cl
H3C. + H3C. → H3C-CH3
Cl. + H3C. → Cl-CH3
- Nitration
Nitration of alkane is made by heating vapours of alkanes and HNO3 at about 400oC to give nitroalkanes.
¨This is also known as vapour phase nitration.

- Combustion:
?Alkanes burn readily with non luminous flame in presence of air or oxygen to give CO2 & water along with evolution of heat.
C2H6 + 7O2 → CO2 +6H2O + heat
- Aromatization
?¨Alkanes having six to 10 carbon atoms are converted into benzene and its homologues at high pressure and temperature in presence of catalyst.

- Oxidization of 30 alkane:?
Tertiary alkanes are oxidized to tertiary alcoholsby KMnO4
R3CH + KMnO4 → R3COH
Alkene (olefins)
- Open chain, Unsaturated hydrocarbons with general formula (CnH2n).
- At least one >c=c< (double bond) group i.e. sp2 hybridisation, is present throughout the chain.
- Allene: alkene molecule in which at least one C has double bonds with each of the adjacent carbon i.e. -c=c=c- group.
- Isomeric with saturated cycloalkanes.

Geometric Isomers:

Z is used if the higher - priority substituents on each C are on the same side of the double bond.letter E is used if they are on opposite sides

Heats of Hydrogenation: Heat of hydrogenation increases with increase in stability of alkene.

Order of heat of hydrogenation: 1-Butene> cis-2-Butene > trans-2-Butene
Order of stability: 1-Butene> cis-2-Butene > trans-2-Butene
Preparation of Alkenes:
1. Cracking of petroleum: 
2. Dehydrohalogenation of alkyl halides: RCH2CH2X + alc.KOH → RCH = CH2
3. Dehydration of Alcohols :
Saytzeff Rule: In dehydration and dehydrohalogenation the preferential order for removal ofan H is 3° > 2° > 1°

4. Reduction of alkynes:

Chemical Properties:
1. Electrophilic Polar Addition Reactions
Reagent
|
Product
| ||
Name
|
Structure
|
Name
|
Structure
|
Halogens
(Cl2, Br2 only)
|
X:X
|
Ethylene dihalide
|
CH2XCH2X
|
Hydrohalic acids
|
H:X
|
Ethyl halide
|
CH3CH2X
|
Hypohalous acids
|
X:OH
|
Ethylene halohydrin
|
CH2XCH2OH
|
Sulfuric acid (cold)
|
H:OSO2OH
|
Ethyl bisulfate
|
CH3CH2OSO3H
|
Water (dil. H3O+)
|
H:OH
|
Ethyl alcohol
|
CH3CH2OH
|
Borane
|
H2B:H
|
Ethyl borane
|
(CH3CH2BH2) → (CH3CH2)3B
|
Peroxyformic acid
|
H:O-OCH=O
(HCO3H)
|
Ethylene glycol
|
CH2OHCH2OH
|
2. Addition of Hydrogen Halides to Alkenes: Markovnikov’s Addition:
R - CH = CH2 + HBr → R – CHBr – CH3
Mechanism:
R - CH = CH2 + HBr → R – CH+ - CH3 +Br-
R – CH+ - CH3 + Br- → R – CHBr - CH3
R – CH+ - CH3 + Br- → R – CHBr - CH3
Anit- Markovnikov’s Addition (Peroxide Effect):
R - CH = CH2 + HBr + (C6H5CO)2O2 → R – CHBr – CH3
Mechanism
Initiation:
R - O - O - R → 2RO.
RO. + HBr → Br. + ROH
Propagation
CH3CH = CH2 + Br. → CH3·CH - CH2Br
CH3·CHCH2Br + HBr→ CH3CH2CH2Br + Br.
Termination:
2RO. → R - O - O - R
Br. + Br.→Br2
3. Addition of Water to Alkenes: Acid Catalyzed Hydration:
Reagent
|
Product
| ||
Name
|
Structure
|
Name
|
Structure
|
Halogens
(Cl2, Br2 only)
|
X:X
|
Ethylene dihalide
|
CH2XCH2X
|
Hydrohalic acids
|
H:X
|
Ethyl halide
|
CH3CH2X
|
Hypohalous acids
|
X:OH
|
Ethylene halohydrin
|
CH2XCH2OH
|
Sulfuric acid (cold)
|
H:OSO2OH
|
Ethyl bisulfate
|
CH3CH2OSO3H
|
Water (dil. H3O+)
|
H:OH
|
Ethyl alcohol
|
CH3CH2OH
|
Borane
|
H2B:H
|
Ethyl borane
|
(CH3CH2BH2)®(CH3CH2)3B
|
Peroxyformic acid
|
H:O - OCH = O
(HCO3H)
|
Ethylene glycol
|
CH2OHCH2OH
|

4. Oxymercuration-Demercuration:

Examples:

5. Hydroboration-Oxidation:

Examples:


6. Halogen Addition in Non-polar Solvent:


7. Halogen Addition in Aqueous Medium:


8. Syn – Hydroxylation: Formation of di-oles.


9. Ozonolysis of Alkenes:

Alkyne
- Saturated open chain hydrocarbon with general formula (CnH2n-2).
- At least one -c≡c- (triple bond) group i.e. sp hybridisation, is present throughout the chain.
- Physical properties of alkynes are similar to those of the corresponding alkenes
Preparation
1. Dehydrohalogenation of vic-Dihalides or gem-Dihalides

2. Dehalogenation of vic-Tetrahalogen Compounds


3. Alkyl Substitution in Acetylene; Acidity of º C-H

4. From Calcium Carbide:
CaC2 +2H2O → Ca(OH)2+ C2H2
5. Kolbe’s Electrolysis:

Chemical Properties
1. Hydrogenation: RC ≡ CCH2CH3 + 2H2 → CH3CH2CH2CH2CH3
2. Hydro-halogenation:
Markovnikov addition: RC≡CH +HBr → RCBr=CH2 +HBr→ RCBr2-CH3
Anti-markovnikov addition: RC≡CH +HBr +peroxide → RCH=CHBr

Aromatic Hydrocarbons:
For being aromatic a hydrocarbon should
- be a cyclic compounds.
- have planarity in geometry.
- have complete delocalization of electrons over ring.
- follow Huckel Rule i.e. number of ?? electrons in ring = (4n+2). :

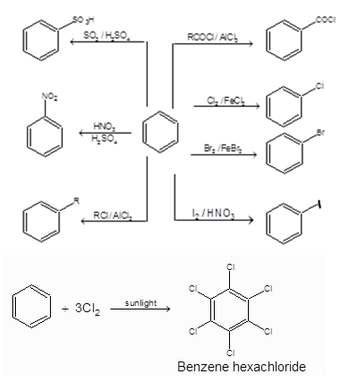
Benzene (C6H6)
1. Structure:

2. Chemical Reactions of Benzene:
Anti-aromatic Hydrocarbons:
Highly unstable compounds.
Number of π electrons in ring = 4n.
Example:

written by arpan ruhil

for ms dh
nyc notes
ReplyDeleteBhut bdya
ReplyDelete