Some Basic Concepts of Chemistry:
Matter:
- Anything that exhibits inertia is called matter.
- Matter has mass and occupy space.
- The quantity of matter is its mass.
Classification of Matter
Based on chemical composition of various substances.
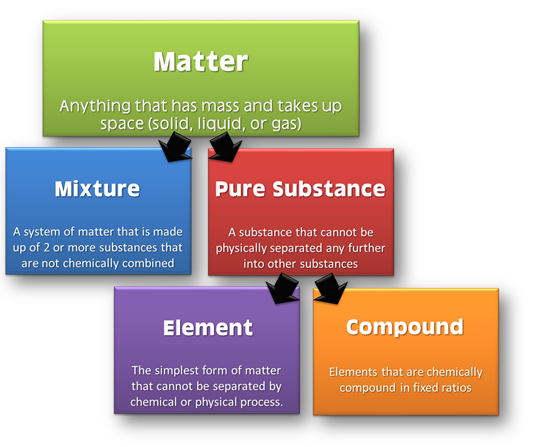
Elements:
- It is the simplest form of the matter.
- Smallest unit of an element is known as atom.
- Total number of the known elements is 118 out of which 98 elements occur naturally and 20 are formed by artificial transmutation.
- Examples: Na, K, Mg. Al, Si, P, C, F, Br etc.
Compound:
- It is a non-elemental pure substance.
- Formed by chemical combination of two or more atoms of different elements in a fixed ratio.
- Examples: H2O, CO2, C6H12O6 etc.
Mixture:
- Formed by physical combination of two or more pure substances in any ratio.
- Chemical identity of the pure components remains maintained in mixtures.
- Homogeneous mixtures are those whose composition for each part remains constant. Example, Aqueous and gaseous solution.
- Heterogeneous mixtures are those whose composition may vary for each and every part. Example, Soil and concrete mixtures.
Physical Quantities and Their Measurement:
Fundamental Units:-
?These units can neither be derived from one another nor can be further resolved into any other units. Seven fundamental units of the S.I. system
Physical quantity
|
Name of the unit
|
Symbol of the unit
|
Time
|
Second
|
S
|
Mass
|
Kilogram
|
kg
|
Length
|
Meter
|
m
|
Temperature
|
Kelvin
|
K
|
Electric current
|
Ampere
|
A
|
Luminous intensity
|
Candela
|
Cd
|
Amount of substance
|
Mole
|
Mol
|
Derived Units:-
These units are the function of more than one fundamental unit
Quantity with Symbol
|
Unit (S.I.)
|
Symbol
|
Velocity (v)
|
Metre per sec
|
ms-1
|
Area (A)
|
Square metre
|
m2
|
Volume (V)
|
Cubic metre
|
m3
|
Density (r)
|
Kilogram m-3
|
Kg m-3
|
Energy (E)
|
Joule (J)
|
Kg m2s-2
|
Force (F)
|
Newton (N)
|
Kg ms-2
|
Frequency (n)
|
Hertz
|
Cycle per sec
|
Pressure (P)
|
Pascal (Pa)
|
Nm-2
|
Electrical charge
|
Coulomb (C)
|
A-s (ampere – second)
|
 Measurement of temperature
Measurement of temperature
Three scales of temperature
- Kelvin scale (K)
- Degree Celsius scale (oC)
- Degree Fahrenheit scale (oF)
?Relations between the scales:
- oF = 9/5(oC) + 32
- K = oC + 273
- 0 K temperatures is called absolute zero.?
Dalton’s Atomic Theory:
- Every matter consists of indivisible atoms.
- Atoms can neither be created nor destroyed.
- Atoms of a given element are identical in properties
- Atoms of different elements differ in properties.
- Atoms of different elements combine in a fixed ratio to form molecule of a compound.
?

Precision and Accuracy:
- Precision: Closeness of outcomes of different measurements taken for the same quantity.
- Accuracy: Agreement of experimental value to the true value
Significant Figures:
 Rules:
Rules:
- All non-zero digits are significant.
- Zeroes preceding the first non-zero digit are not significant.
- Zeroes between two non-zero digits are significant.
- Zeroes at the end of a number are significant when they are on the right side of the decimal point.
- Counting numbers of objects have infinite significant figures.
Scientific Notation:
Numbers are represented in N × 10n form.
Where,
N = Digit term
n = exponent having positive or negative value.
Examples,
12540000 = 1.254 × 107
0.00456 = 4.56 ×10-3
Mathematical Operations of Scientific Notation:
Multiplication and Division:
Follow the same rules which are for exponential number.
Example: (7.0 ×103 ) × (8.0×10-7 ) = ( 7.0×8.0) × ( 10[3 + (-7)] ) = 56.0 × 10-4
Result cannot have more digits to the rite of decimal point than either of the original numbers
(7.0 ×103 ) / (8.0×10-7 ) = ( 7.0/8.0) × ( 10[3 - (-7)] ) = 0.875 ×1010 = 0.9 ×1010
Addition and Subtraction:
Numbers are written in such way that they have same exponent and after that coefficients are added or subtracted.
(5 ×103 ) + (8×105 ) = (5 ×103 ) + (800×103 ) = (5+800) ×103 = 805 ×103
Result must be reported with no more significant figures as there in the original number with few significant figures.
Rules for limiting the result of mathematical operations:
- If the rightmost digit to be removed is more than 5, the preceding number is increased by one.
- If the rightmost digit to be removed is less than 5, the preceding number is not changed.
- If the rightmost digit to be removed is 5, then the preceding number is not changed if it is an even number but is increased by one if it is an odd number.
Dimensional Analysis:-
- This is based on the fact that ratio of each fundamental quantity in one unit with their equivalent quantity in other unit is equal to one.
- Derived unit first expressed in dimension and each fundamental quantities like mass length time are converted in other system of desired unit to work out the conversion factor
- Original Quantity × Conversion factor = Equivalent Quantity
(In former unit) (In final Unit)
Example:- (1 kg/2.205 pound) = 1 = (1kg/1000gm)
So 1 kg = 2.205 pound = 1000 gm
Laws of Chemical Combination:

- Law of conservation of mass: “For any chemical change total mass of active reactants are always equal to the mass of the product formed”
?

- Law of constant proportions:
“A chemical compound always contains same elements in definite proportion by mass and it does not depend on the source of compound”.
 ?
?- Law of multiple proportions:
“When two elements combine to form two or more than two different compounds then the different masses of one element which combine with fixed mass of the other element bear a simple ratio to one another”

- Law of reciprocal proportion:
“ If two elements B and C react with the same mass of a third element (A), the ratio in which they do so will be the same or simple multiple if B and C reacts with each other”.
?

- Gay Lussac’s law of combining volumes:
“At given temperature and pressure the volumes of all gaseous reactants and products bear a simple whole number ratio to each other”.

Atomic and Molecular Masses:
Atomic Mass:
- It is mass of an atom.
- Reported in atomic mass unit “amu” or unified mass “u”
- One atomic mass unit i.e. amu, is the mass exactly equal to one-twelfth the mass of one carbon-12 atom.
Molecular Mass:
- It is mass of a molecule of covalent compound.
- It is equal to the sum of atomic masses of all the elements present in the molecule.
- For example, Molecular mass of H2O = 2(Atomic mass of H) + Atomic mass of O
Formula Unit Mass
- It is mass of a molecule of an ionic compound.
- It is also equal to the sum of atomic masses of all the elements present in the molecule
Mole Concept:
Mole:
- It is unit of amount of substance.
- One mole amount of substance that contains as many particles or entities as there are atoms in exactly 12 g of the 12C isotope.
?

Molar mass:
- It is mass of one mole of a substance in gram
- Molar mass in gram in numerically equal to atomic/molecular/formula mass in amu or u.
?Percentage Composition:
Mass percentage of an element in a compound = (Mass of that element in the compound / Molecular mass of the compound) ×100
Percentage yield:
- It is the ratio of actual yield of the reaction to the theoretical yield multiplied by 100.

Empirical formula and Molecular Formula:

- Molecular Formula:- The formula which represents the actual number of each individual atom in any molecule is known as molecular formula.
- Empirical Formula:- It expresses the smallest whole number ratio of the constituent atom within the molecule.
?


also,

Limiting Reagent:
It is the reactant which is totally consumed during the course of reaction and when it is consumed reaction stops.
For a balanced reaction:
A +B → C + D
B would be a limiting reagent if 

Similarly, A is a limiting reagent if 

Concentration of the Solutions:

- Mass by Mass Percentage: -
?Amount of solute in gram present per 100 gm of the solution.
Mass percentage of solute =
Mass percentage of solute =

- Mass by Volume Percentage:-
?Amount solute in gram present per 100 mL of the solution.
- Volume by Volume Percentage:-
?Volume of solute per 100 mL of the solution
Volume by volume percentage of solute =
Volume by volume percentage of solute =

- Parts per million ( ppm) :-
The amount of solute in gram per million (106) gram of the solution.
ppm =
ppm =

- Mole fraction:-
Ratio of the moles of one component of the solution to the total number of moles of solution
Total mole fraction of all the components of a solution is equal to 1.
For binary solutions having two components A and B
Total mole fraction of all the components of a solution is equal to 1.
For binary solutions having two components A and B
Mole fraction of A

Mole fraction of B

or XB = 1- XA
- Molarity(M):-
Number of moles of solute per 1000 mL of the solution.


- Molality(m):-
?Number of moles of solute per 1000 gram of the solvent.
?

good notes
ReplyDeleteNice
ReplyDeletelage raho bhai
ReplyDelete